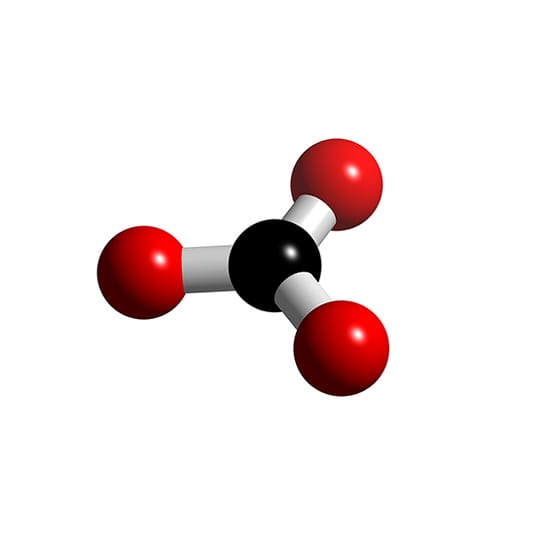
Co3 2 Molecular Geometry
CO3 2- Lewis Structure Step-by-Step Guide 1. Determine the total number of valence electrons In the carbonate ion (CO3^2-), carbon (C) contributes 4 valence electrons, while each oxygen (O) atom contributes 6 valence electrons. Since there are three oxygen atoms, the total number of valence electrons is:

SOLVED Draw the Lewis structure of CO. What is the electron geometry, molecular shape, and
I quickly take you through how to draw the Lewis Structure of CO3 2- (Carbonate Ion). I also go over the resonance, hybridization, shape and bond angle.

What is the molecular and electron geometry of \ce{CO3^{2} Quizlet
1. Count the total valence electrons in [CO3]2- The Lewis dot structure of a molecule is referred to as a simplified representation of all the valence electrons present in it. Therefore, the very first step while drawing the Lewis structure of [CO 3] 2- is to count the total valence electrons present in the concerned elemental atoms.
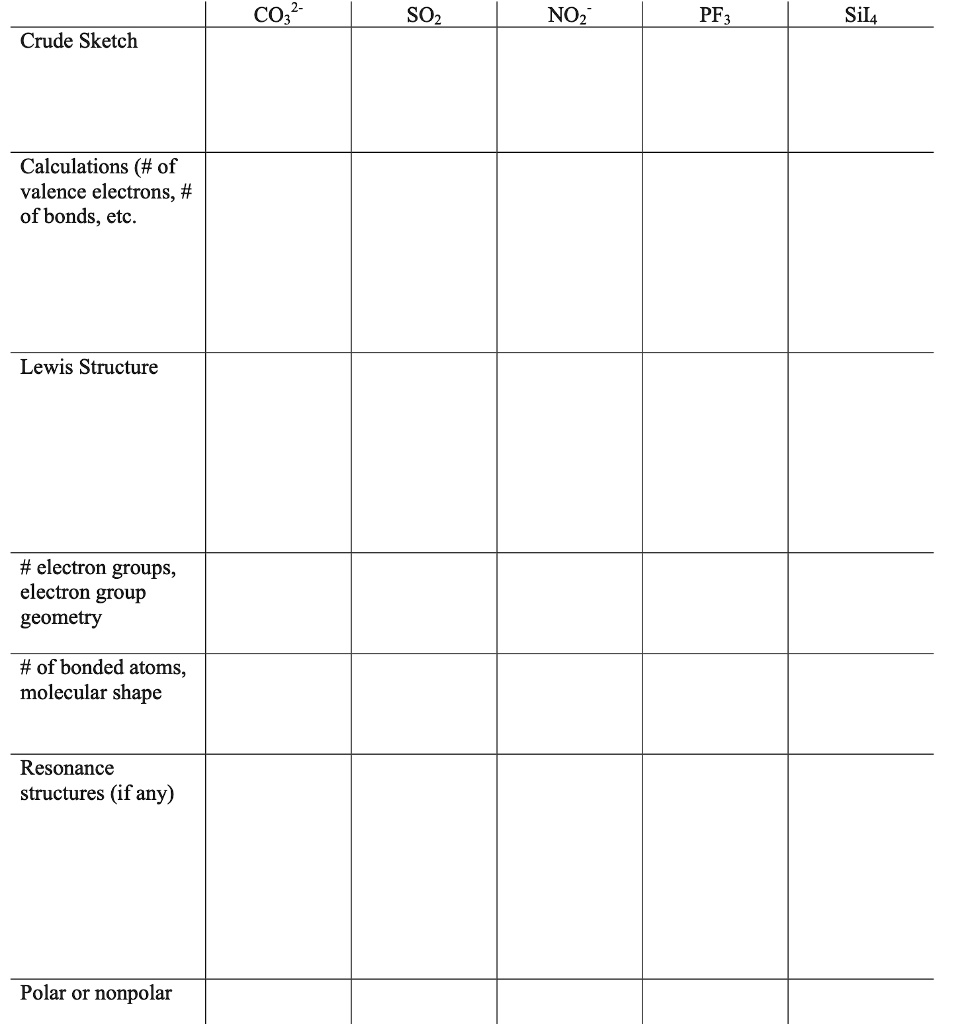
SOLVED CO3 SOz NOz PFz Sil4 Crude Sketch Calculations ( of valence electrons, of bonds, etc
Step - 8 Last is to determine shape, hybridization and bond angle of CO32- lewis structure. CO32- lewis structure.. Carbonate (CO32-) ions have 2- negative formal charge and also it has quite sufficient lone electron pairs present on three O atoms out if which two O atoms have -1 negative charge. Thus it can easily gain or accepts H+ ions.
CO23. Solutions to Selected Problems, CO19 Chemistry LibreTexts
3812-32-6 Carbonate ions Karbonat View More. Molecular Weight 60.009 g/mol Computed by PubChem 2.2 (PubChem release 2021.10.14) Parent Compound CID 767 (Carbonic Acid) Dates Create: 2005-08-08 Modify: 2024-01-06 Description Carbonate is a carbon oxoanion. It is a conjugate base of a hydrogencarbonate. ChEBI
PPT Lecture 11 VSEPR Theory, Molecular Shape PowerPoint Presentation ID6303965
A step-by-step explanation of how to draw the CO3 2- Lewis Dot Structure (Carbonate ion). For the CO3 2- structure use the periodic table to find the total number of valence electrons.
How To Draw Resonance Structures Foreversalary
Hello Guys!CO32- ion comprises one Carbon atom and three Oxygen atoms along with two additional electrons. In this video, we find out the molecular geometry.

CO3 2 Lewis Structure & Geometry YouTube
The first step in drawing the CO 3 2-Lewis structure is to determine the total number of valence electrons in the molecule. This can be calculated by multiplying the valence electrons of each atom. Carbon is located in group 14 of the periodic table and has four valence electrons, while oxygen, belonging to group 16, has six valence electrons. In CO 3 2-, which consists of one carbon atom and.

How to calculate bond order of co3^ 2? Brainly.in
Figure 5.2.9 5.2. 9: (a) H2O has four regions of electron density around the central atom, so it has a tetrahedral electron-pair geometry. (b) Two of the electron regions are lone pairs, so the molecular structure is bent. Exercise 5.2.3 5.2. 3. The hydronium ion, H 3 O +, forms when acids are dissolved in water.

Lewis dot structure for NF3.and CO3^2_ Brainly.in
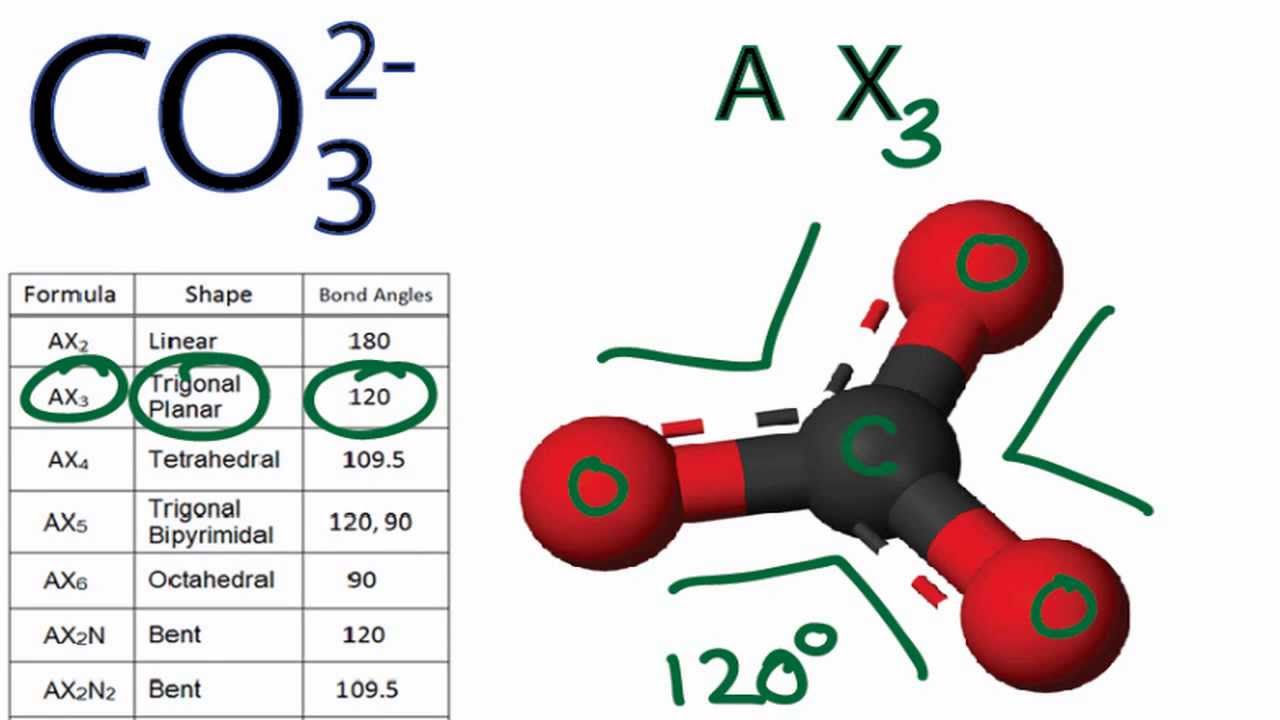
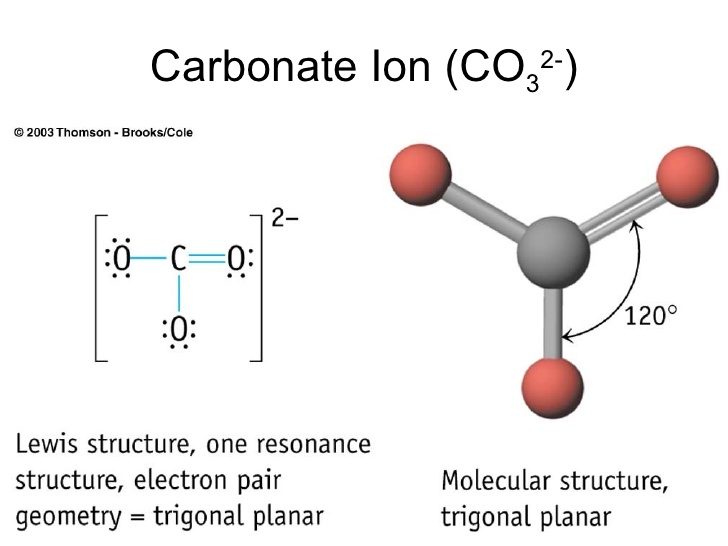
The shape of the CO3 (2-) molecule is trigonal planar, which means that the molecule is symmetrical and the bond dipoles cancel out. This results in a non-polar molecule overall, even though each bond is polar. The polar bonds cancel out each other's dipole moment due to the symmetry of the molecule, resulting in a net dipole moment of zero.

what is the shape of SO3 , CO3^2, NO3^1plx explain how with Lewis dot structure .. m very
1.8K 161K views 3 years ago This chemistry video tutorial explains how to draw the lewis structure of CO3 2- also known as the carbonate ion. This video discusses the resonance structure of.
Co Formulacion SEO POSITIVO
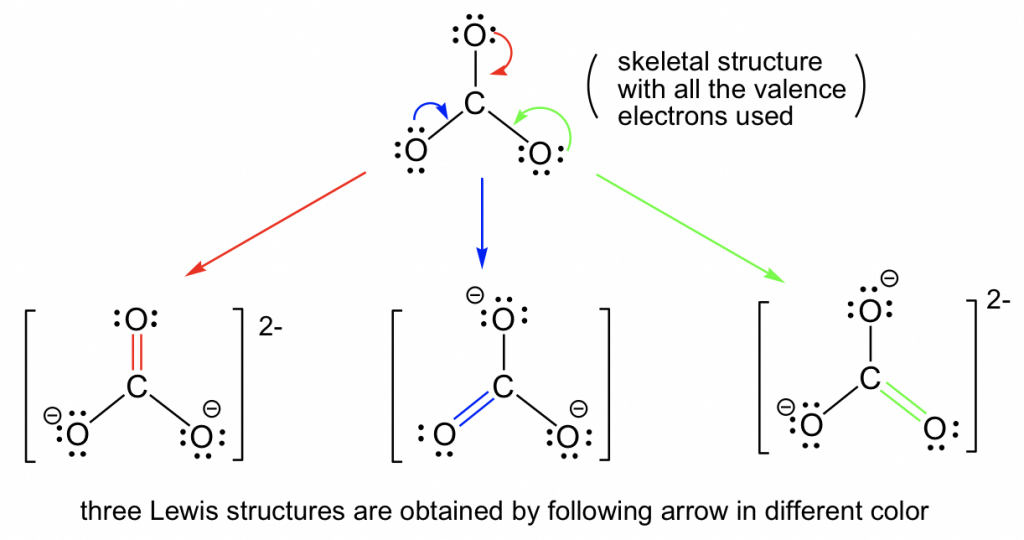
CO32- Geometry and Hybridization - Chemistry Steps Examples Geometry CO32- Geometry and Hybridization There are 4 + 3×6 + 2 = 24 electrons. The carbon goes in the middle, and the oxygens take 6 electrons each as three lone pairs: The carbon lacks an octet, so we use a lone pair from one oxygen to make a double with it.
What are some examples of trigonal sp^2 hybrids? Socratic
329 Share 110K views 10 years ago A quick explanation of the molecular geometry of CO3 2- including a description of the CO3 2- bond angles..more.more A quick explanation of the.

[Download 35+] Possible Resonance Structures For Co32
Step 1: Count the Total Number of Valence Electrons. In CO32- ion, we have one carbon atom and three oxygen atoms along with two negatively charged electrons carrying the charge. Valence electrons refer to the number of electrons in the outermost shell of an atom around the nucleus that help in determining the valency of the given atom.

Co3 2 Molecular Geometry
The Lewis structure of H 2 O indicates that there are four regions of high electron density around the oxygen atom: two lone pairs and two chemical bonds: Figure 4.3.9 4.3. 9. Thus, the electron-pair geometry is tetrahedral and the molecular structure is bent with an angle slightly less than 109.5°.

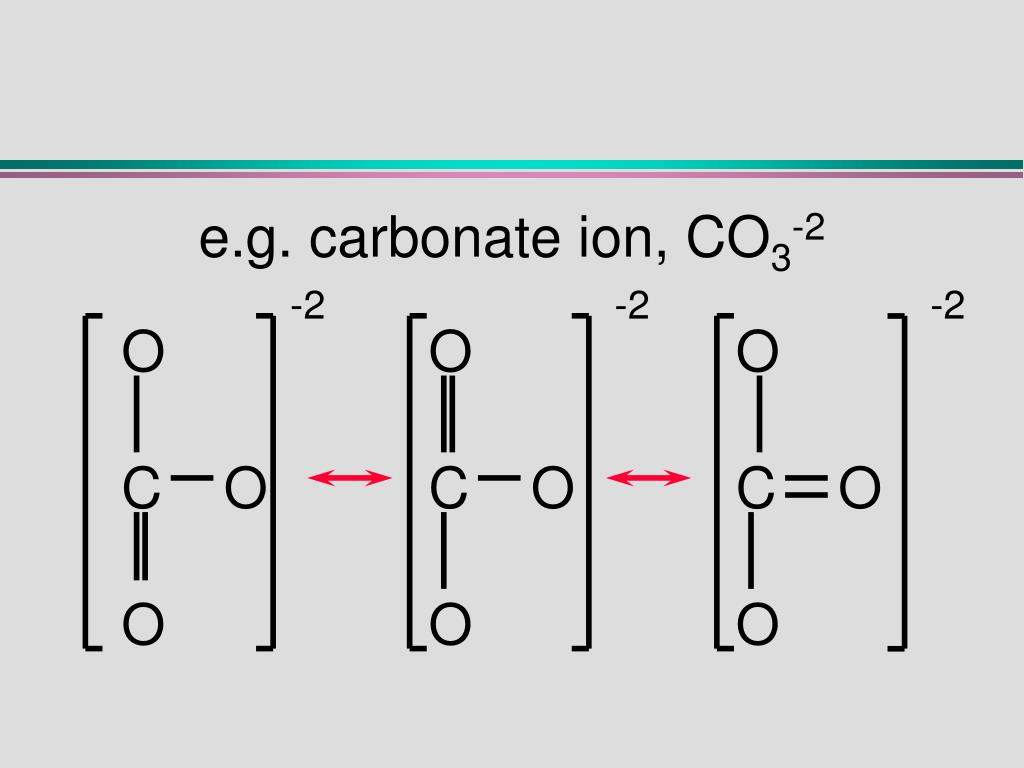
Draw the resonance structure of 1) CO3 2 2) Benzene 3) CO2 4) O3 Pls Answer It ASAP . I need
Lewis structure of CO3 2- contains one double bond and two single bonds between the Carbon (C) atom and Oxygen (O) atom. The Carbon atom (C) is at the center and it is surrounded by 3 Oxygen atoms (O). Both the single bonded Oxygen atoms (O) have -1 formal charge. Let's draw and understand this lewis dot structure step by step.